Four key things to keep in mind when using the simulations
Your approach to using the simulations will determine how much you get out of them. The simulations are designed to help you learn through exploration, and so HOW MUCH and HOW DEEPLY you explore will affect how well you understand kinetic molecular theory.
Think about the particles moving around the container in the model. Think about some of the stories/narratives you read on the site and think about how the explanatory model you are using in the simulation help you to understand the phenomenon presented in the story. We could just explain the ideas behind KMT to you and leave it at that, but then we’d be no different than a textbook. To make the most of these simulations, you should explore, observe, think about what you are seeing and try to make connections as much as possible.
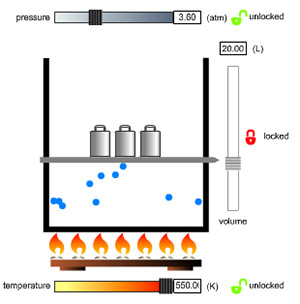
First, observe carefully and ask yourself what you are seeing. Are the particles all the same? What are the particles doing? What happens to the graph when I change this setting? Remember, the simulation is simplified to make it easy to see things, so look!
Second, try to discover relationships between the simulation variables. For example, if you increase one, what happens to the other? What does that relationship look like on the graph? The relationships you are able to discover are probably the relationships we’d like you to learn.
Third, pay attention to what happens to the particles as you manipulate the variables. Are they speeding up or slowing down? Are they bunching together or spreading out? Think about what is causing those changes, and what those changes would mean in real-life example.

Fourth, try to link what you see in the simulations to what you know of the real world. If decreasing the temperature in the simulation does something to the gas particles in a closed container, can you think of an example of a closed container being placed in the refrigerator? Remember, the reason we learn about kinetic molecular theory is because it helps us understand why things happen the way they do in the observable world, so you should try hard to relate KMT to what you see every day.